Cureline Group
A global translational and precision medicine CRO providing HBS biobanking and laboratory services for accelerated drug R&D and Dx development since 2003
- Standard and custom protocols and services
- Global clinical network, regulatory expertise, logistics
- Precision medicine expertise in major TA
- Collaborative approach to project management
- HBS Biorepository in San Francisco Bay area (USA)

What We Do

Cureline headquarters
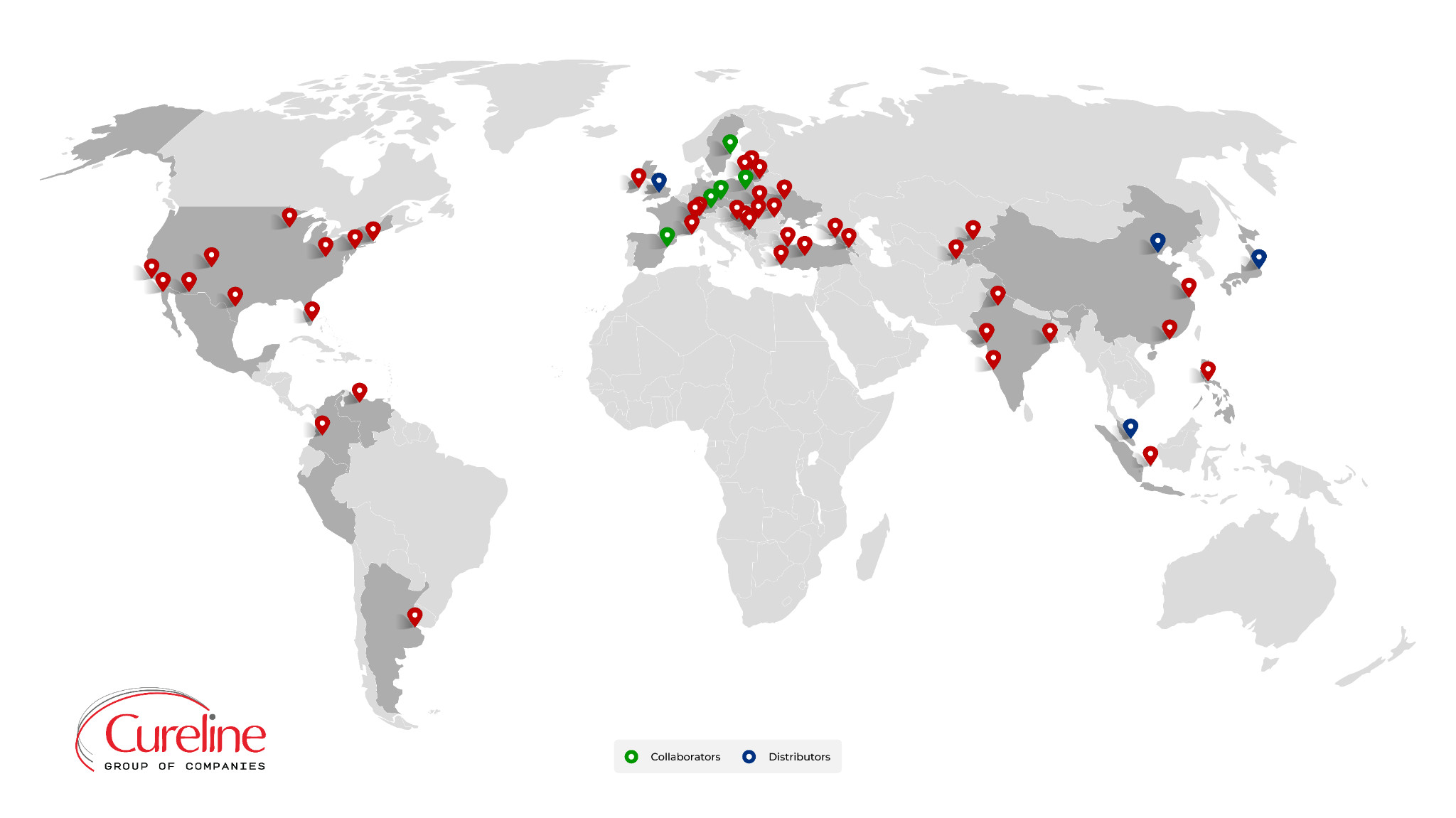
Cureline was originated in the San Francisco Bay area (California, USA) in 2003.
Together with our global clinical network and business partners in Europe, Asia, Africa and the Americas, Cureline Group provides comprehensive global biobanking and HBS analyses services.
Since 2003 we have partnered with 150+ validated clinical sites, completed projects for 700 satisfied clients, and participated in two major scientific NIH (USA) consortiums, the TCGA and the CPTAC.